CELL ENCAPSULATION
Body Armour for Living Cells: protects, shields and extends the life of living cells
What’s new?
Avant Technologies and Austrianova Secures Exclusive License for Klotho Producing Cell Line to Advance Anti-Aging Therapies
19 November 2025

Micro-encapsulation of living cells for immuno-protection and localization in patients

Encapsulation of probiotic bacteria and yeast for stomach acid protection and ambient storage
Our Services
To find out more about our unique award winning protective cell encapsulation technology and our cell biology expertise, click a panel on the right.
Encapsulated
Cell-in-a-box
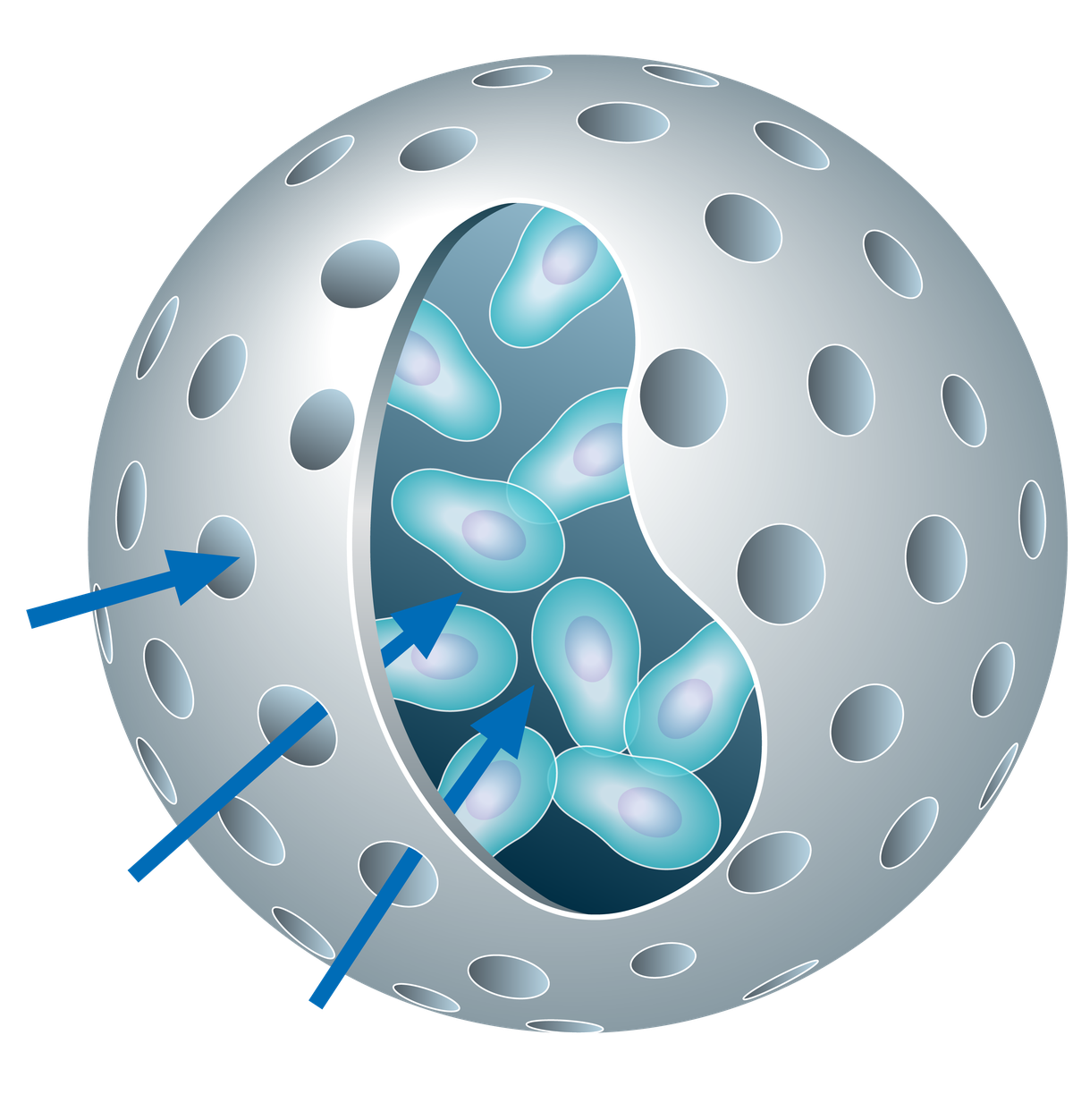
The Cell-in-a-Box® technology builds a porous capsule round the cells, allowing free diffusion of nutrients in, and waste products out, and also allowing any biological molecules synthesized by the cells to escape.
BAC-in-a-box
Bac-in-a-Box® technology, developed by Austrianova, is a means to protect, isolate, store and transport living bacteria and yeast.
Bac-in-a-Box® technology builds a porous capsule round the bacteria or yeast, allowing free diffusion of nutrients in, and waste products out, and also allowing the bacteria and yeast to grow in the capsules.
They can then be released from the capsule.
Non Encapsulated
STEM CELL MANUFACTURING
CELL LINE DEVELOPMENT
GMP CELL BANKING (GMP4Cells)
News and Recent Publications
Avant Technologies and Austrianova Secure Exclusive License for Klotho Producing Cell Line to Advance Anti-Aging Therapies
LAS VEGAS, NEVADA, November 18, 2025 – Avant Technologies, Inc. (OTCQB: AVAI) (“Avant” or the “Company”), an emerging biotechnology company focused on developing cell-based therapies for diabetes and age-related disorders, today announced that its 50/50 joint venture...
Avant Technologies and Austrianova Sign Joint Venture, Licensing Agreement to Advance Klotho-Based Therapies
LAS VEGAS, Sept. 18, 2025 /PRNewswire/ -- Avant Technologies Inc. (OTCQB: AVAI) ("Avant" or the "Company"), a Nevada-based corporation, and Austrianova (SGAustria Pte. Ltd.), a Singapore-based biotechnology leader, today announced the formation of a Joint Venture and...
Contact us
Email Us